The Quantum Mechanical Model Is Used to Describe
Quantum mechanical model of an atom. Modern atomic model quantum mechanical model this model describes the probability an electron will be found in a certain location.
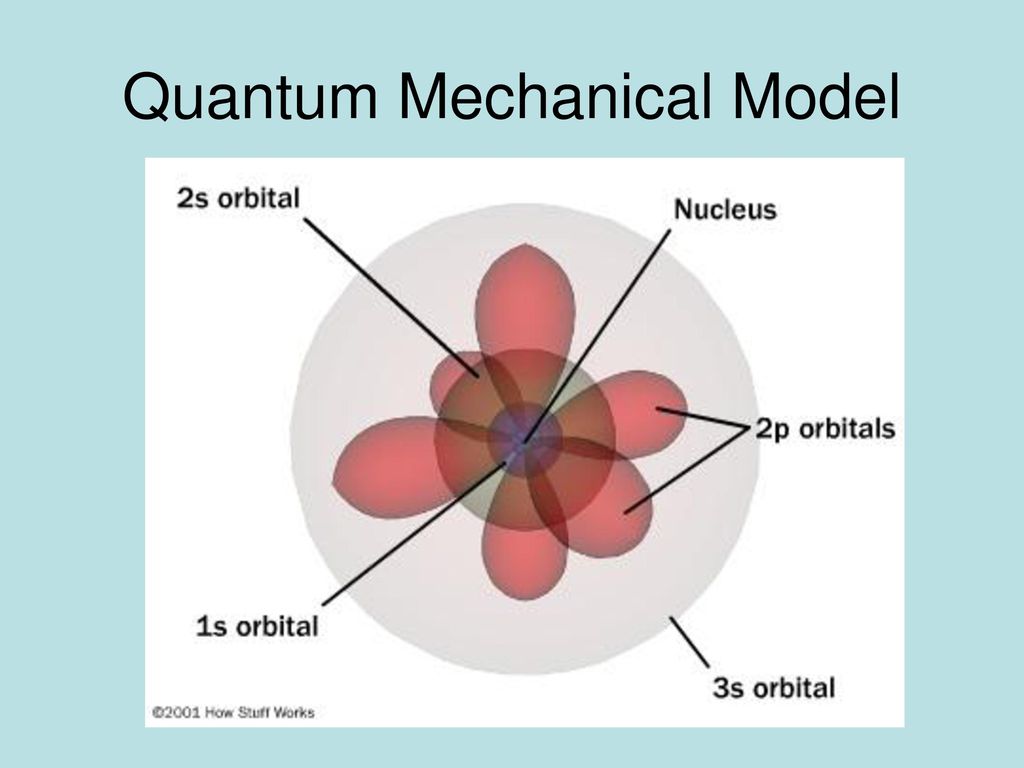
Quantum Mechanical Model Of The Atom Orbitals And Electron Configuration Mrs Hayes Chemistry Ppt Download
Quantum Mechanical Model of Atom.
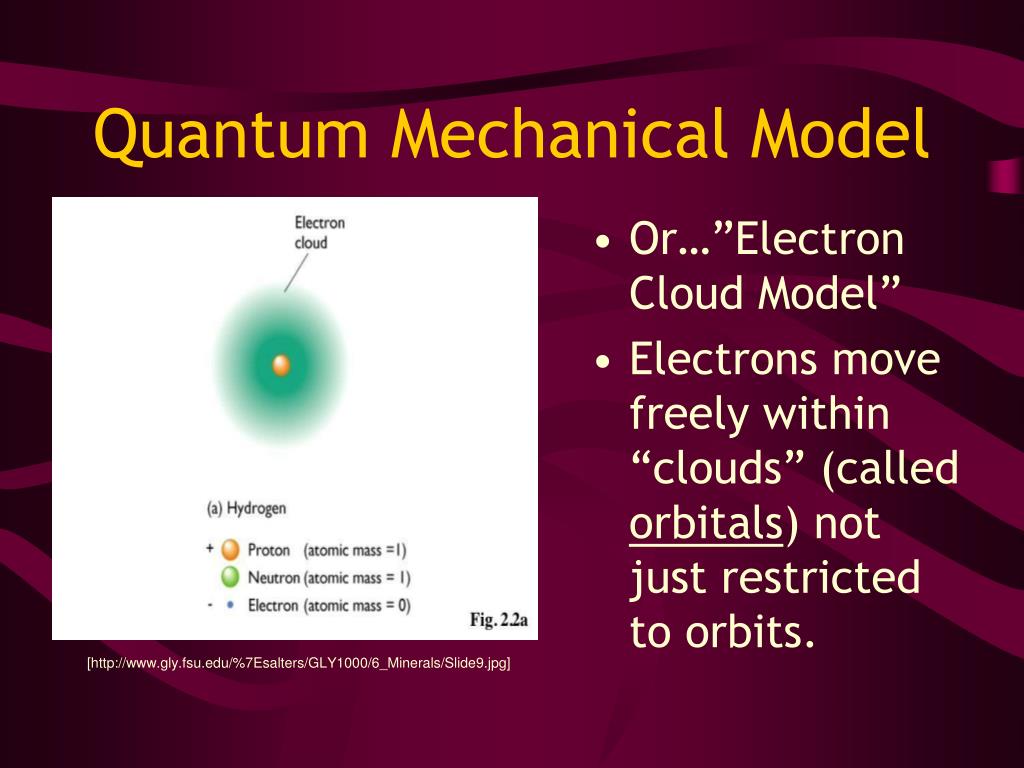
. Quantum mechanics is based on Schrödingers wave equation and its solution. Electron spin and the Stern-Gerlach experiment. The quantum mechanical model of an atom describes the probability of finding electrons within given orbitals or three-dimensional regions of space within an atom.
A mathematical equation is used to describe the location and energy of the electron in an atom. Quantum mechanical model The quantum mechanical model describes the probable location of electrons in atoms by describing. Schrodinger replaced Bohrs orbits by complex 3-dimensional mathematical wave equations.
A model that explains the behavior of absolutely small particles such as electrons and photons. Question 4 1 Point You have been tasked to give a report about the differences in the Bohr Model v. Google Classroom Facebook Twitter.
This atomic model is known as the quantum mechanical model of the atom. The Bohr model and the quantum mechanical model. Introduction to the quantum mechanical model of the atom.
Because it is used to describe the energy of electrons II. Although it is more difficult to understand than the Bohr model it can be used to explain observations made on complex atoms. Because it is used to determine the directions of the electrons within the orbitals a.
Up to 24 cash back Schrödinger used mathematical equations to describe the likelihood of finding an electron in a certain position. Describe the 5 main differences between the Bohr Atom Model and the Quantum Mechanical model Use the editor to format your answer Question 5 1 Point An element has 4 neutrons 3 electrons and 4 protons. Because it is used to determine the energy within the orbitals IV.
The energy and most likely location of an electron the energy and the most likely location of. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. The Quantum Mechanical Model is used to describe.
A mathmatical function that represents the total probability of finding the electron within a thin spherical shell at a distance r from the nucleus. The quantum mechanical model of an atom uses atomic orbitals to describe the location and shape of the orbital in which the electron is present. Two models of atomic structure are in use today.
Thinking about electrons as probabilistic matter waves using the de Broglie wavelength the Schrödinger equation and the Heisenberg uncertainty principle. Why do you think the quantum mechanical model of atom is important. The quantum mechanical model is based on mathematics.
Unlike the Bohr model the quantum mechanical model does not define the exact path of an electron but rather predicts the odds of the location of the electron. Electrons fill an atoms orbital in order of increasing energy lowest energy first Pauli Exclusion Principle. Quantum Mechanical Model of the atom.
The quantum mechanical model of the atom. The probability of finding an electron at a point within an atom is proportional to the ψ 2 at that point where ψ represents the wave-function of that electron. The Quantum-Mechanical QM model first developed by Erwin Schrodinger in 1926 describes electrons mathematically as both waves and particles.
The solution of the wave equation brings the idea of shells sub-shells and orbitals. The quantum mechanical model of the atom comes from the solution to Schrödingers equation. Because it is used to describe the position of electrons III.
Quantization of electron energies is a requirement. The electron is treated as a wave around the nucleus of an atom. In 1926 Austrian physicist Erwin Schrödinger 18871961 used the wave-particle duality of the electron to develop and solve a complex mathematical equation that accurately described the behavior of the electron in a hydrogen atom.

Quantum Mechanical Model Youtube
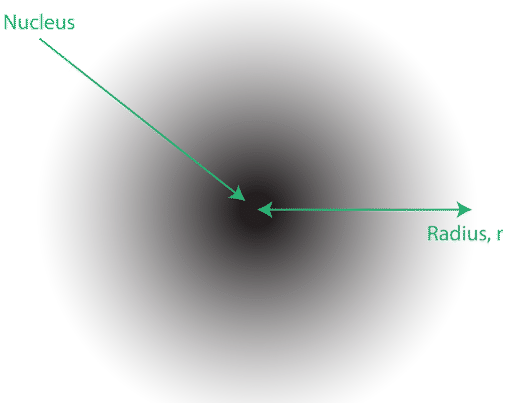
5 11 Quantum Mechanical Atomic Model Chemistry Libretexts

Models Of The Atom Mechanical Model Quantum World Physical Science
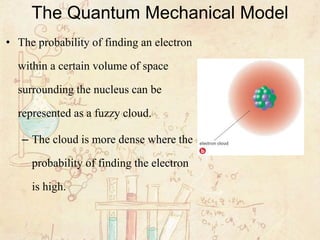
The Quantum Mechanical Model Of The Atom

The Quantum Mechanical Model Ck 12 Foundation
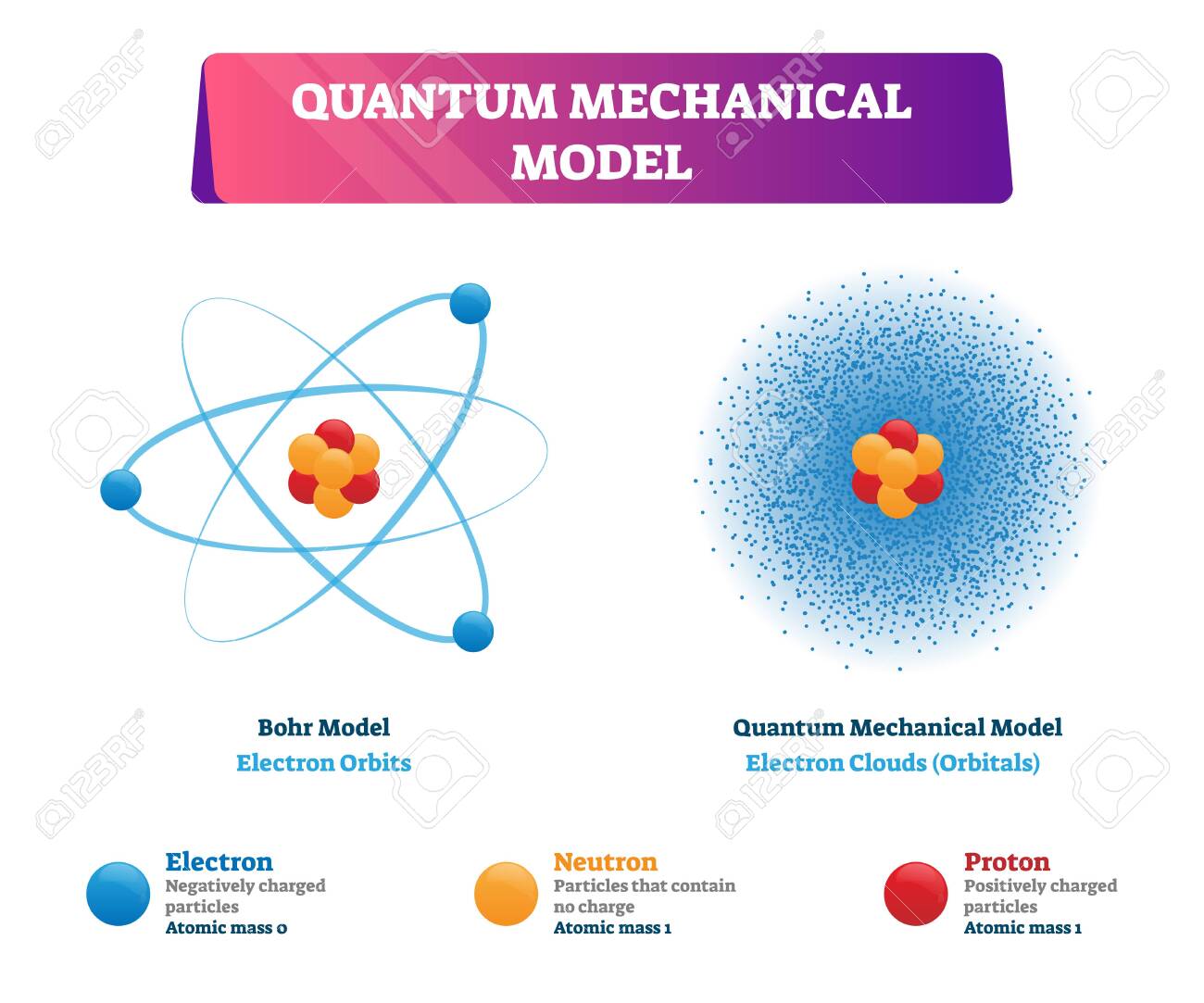
Quantum Mechanical Model Vector Illustration Physics Examples Negatively Charged Electron Neutron And Positively Charged Proton In Bohr Model As Electron Orbits And Quants Model As Electron Clouds Royalty Free Cliparts Vectors And

The Quantum Mechanical Model Definition Overview Video Lesson Transcript Study Com

Ppt Quantum Mechanical Model Powerpoint Presentation Free Download Id 4977042

Quantum Mechanical Atomic Model Ck 12 Foundation
The Quantum Mechanical Model Of The Atom Article Khan Academy

The Quantum Mechanical Model Of The Atom Ppt Download
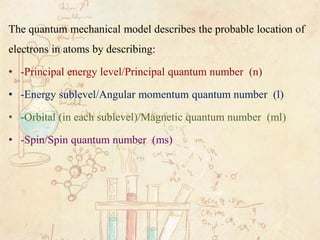
The Quantum Mechanical Model Of The Atom

Quantum Numbers And Quantum Mechanical Model Diagram Quizlet

Physical Science Quantum Mechanics Britannica
Quantum Mechanical Model Of The Atom Orbitals And Electron Configuration Mrs Hayes Chemistry Ppt Download

The Quantum Mechanical Model Definition Overview Video Lesson Transcript Study Com

Why Does The Quantum Mechanical Model Predict More Bright Lines Than The Bohr Model Quora
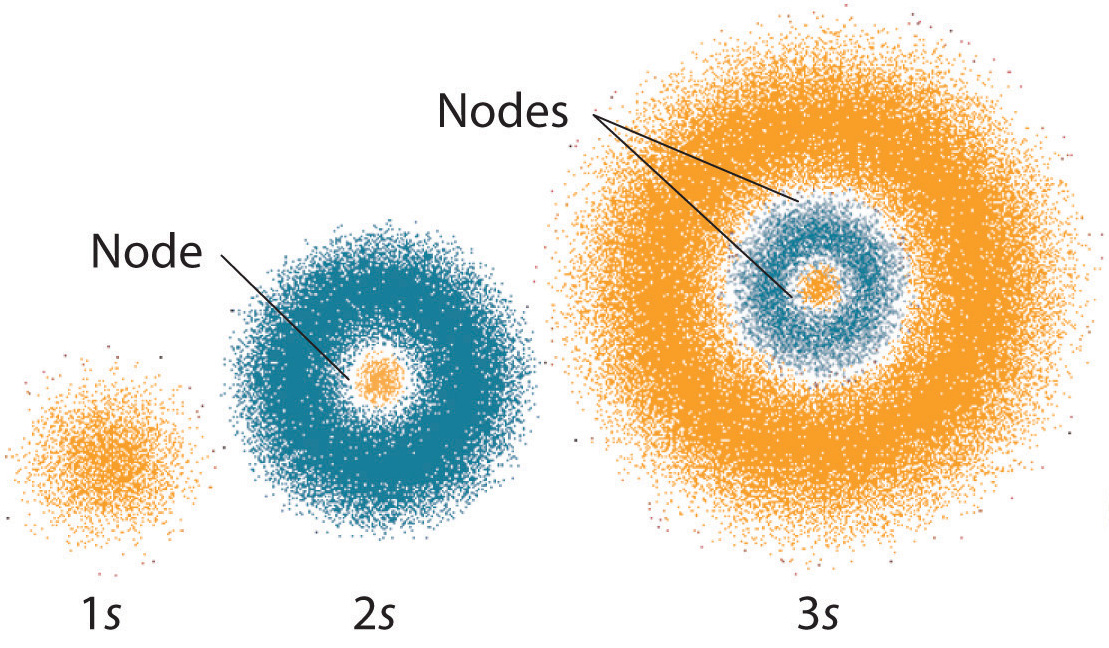
The Quantum Mechanical Model Of The Atom Article Khan Academy

Comments
Post a Comment